21+ Calculate Zeff For A Valence Electron In An Oxygen Atom.
S orbitals shield the electrons from the nucleus more than p-orbitals which shield more in d. Nickel atom can lose two electrons to.
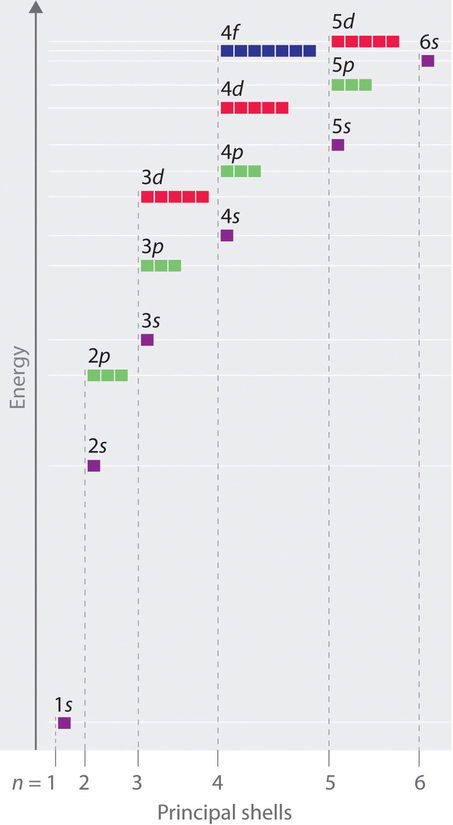
7 8 Electron Configurations Chemistry Libretexts
Energy is released when an atom gains an electron Explanation.

. 15999 g 294305 gmol mol O 1 mol C14 H18 N 2 O 5 340 102 mol C14H18N2O5 2943 g C14 H18 N 2 O 5 2943 g 459 g C14H18N2O5 mol 1g. Web Enter the email address you signed up with and well email you a reset link. In a multi-electron atom which orbital will have the highest energy.
The closer an atom is to having a half-full valence shell the higher the melting point high. 100 g C14H18N2O5 c. There are 15 atoms in the first model 11.
Web Enter the email address you signed up with and well email you a reset link. They are two electrons short of a noble has configuration and tend to react to gain those two electrons forming 2- anions. The Na ion is larger than the parent Na atom because the additional electron produces a 3s 2 valence electron configuration while the nuclear charge remains the same.
Bohr was the first scientist to propose the idea that the electron in a hydrogen atom is quantized leading to discrete energies. Web Transcript 10 mol F2 440 g CO2 40 g H2 146 g SF6 34. 19 - 15 4 so the other models oxygen is the fifth atom in the file Align two structures on top of each other using.
NO3- nitrate ion b. 3 and 6 The valence electrons in an oxygen atom are attracted to the nucleus by a positive charge nearly double that of boron. Web In the Bohr model of the hydrogen atom the energy required to excite an electron from n 2 to n 3 is _____ the energy required to excite an electron from n 3 to n 4.
NO2- nitrite ion c. 156 mol d. 3 and 6 On the basis of periodic trends choose the larger atom from each pair if possible.
8 O 1s2 2s2 2p4. Cesium has an atomic number of 55 indicating it has 55 protons and 55 electrons in the neutral atom. Web In a multi electron atom the orbitals in a sub level are degenerate.
From the above conversion factors you can show that 1 m 3 1 10 3 L. Calculate the number of neutrons from the mass number and the number of protons. E ψ² describes the size of an atom.
An oxoanion containing one more oxygen atom than the. Web Estimate the approximate Zeff felt by a valence electron of boron and oxygen respectively. SO32- sulfite ion d.
The ion with the electron configuration of 1s²2s²2p⁶3s²3p⁶3d³ has a total of 21 electrons. Web In this case m3 the oxygen atom is 0 19. SO42- sulfate ion e.
Web A ψ² describes the probability of finding an electron in space. Web nuclear charge Zeff experienced by the electron present in them. 50 mg e.
Web A number of polyatomic anions containing oxygen atoms are named based on the root word of the central or non-oxygen atom and the suffix ate for the one with more oxygen atoms and ite for the one with less oxygen atom. The mass number of an atom is representative of the number of protons and neutrons. C ψ² describes the electronic structure of the atom according to the Bohr model.
31. Web TITRIMETRIC MTHODS Titrimetric methods are widely used in chemistry to determine oxidants reductants acids bases metal ions etc. 14 mol C H 12011 g 10079 g 14007 g 18 mol H 2 mol N mol C mol H mol N 5 mol O b.
Therefore 7 m 3 would equal 7 10 3 L which is close to the answer. Of the options given the only ion with the same. Web The symbol 133Cs133Cs indicates that the mass number is 133.
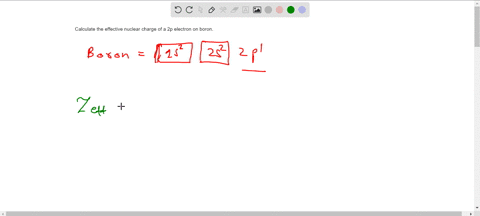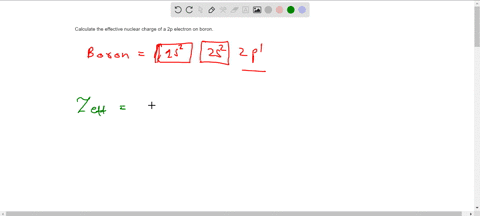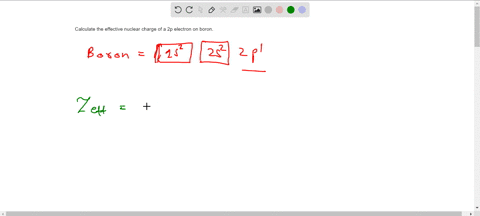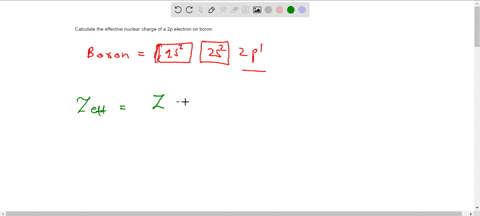
The effective nuclear charge Zeff. Titration is based on a reaction between the analyte unknown sample and the regent of. Web TITRIMETRIC MTHODS Titrimetric methods are widely used in chemistry to determine oxidants reductants acids bases metal ions etc.
Titration is based on a reaction between the analyte unknown sample and the regent of. Show the distribution of electrons in oxygen atom atomic number 8 using. Zeff is the effective nuclear charge the charge that leads.
Web definition of - senses usage synonyms thesaurus. Web Estimate the approximate ZeffZeff felt by a valence electron of boron and oxygen respectively. Greater than When an electron in excited energy level drops to.
B ψ² describes the exact path of electron motion in an orbital. Web The Na ion is significantly smaller than the neutral Na atom because the 3s 1 electron has been removed to give a closed shell with n 2. The addition of one or more electrons to the valence shell Explanation.
Place the lowest energy sublevel at the bottom of the list. An elemental symbol surrounded by dots that represent that atoms valence electrons is known as an _____. That is the oxygen is the first atom in the the first model from PubChem and the 5th atom in the second model from NCICADD.
A chilled steel rod at 200 C is placed in the water. If the final temperature of the system is 2130 C what is the mass of the steel bar. Web Rank the sublevels for a particular principal energy level in order of decreasing energy for a many-electron atom.
D ψ² describes the exact volume of an atom. Electron affinity EA is defined as the energy change for the process of adding an electron to a gaseous atom to form an anion negative ion. A neutral atom of Al has 3 valence electrons.
The oxygen family group 6A has six valence electrons and has an outer electron configuration of ns2np4. 133 55 x 78 x. Web In two chemical reactions 40 grams of oxygen combine with 30 grams of carbon to form one compound and 80 grams of oxygen combine with 3 grams of carbon to form another.

Solved Which Would Experience A Higher Effective Nuclear Chegg Com

Solved 3 Calculate Zeff For Oxygen Atom On Outermost Shell Chegg Com
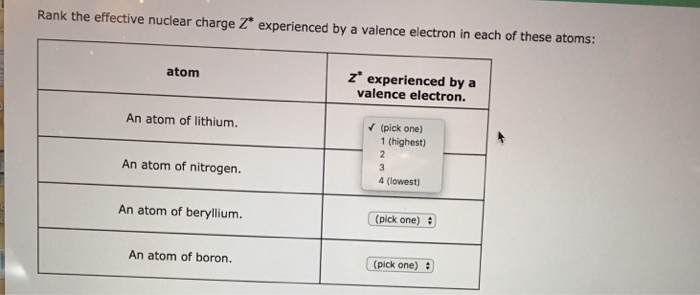
Solved Rank The Effective Nuclear Charge Z Experienced By A Chegg Com

Calculate Zeff For A Valence Electron In An Oxygen Atom Brainly Com
How To Find The Effective Nuclear Charge Of Oxygen Quora
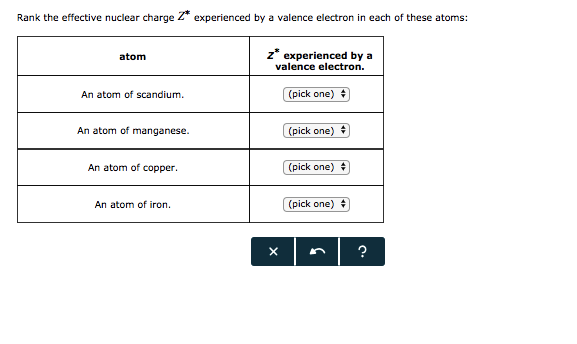
Solved Rank The Effective Nuclear Charge Z Experienced By A Chegg Com
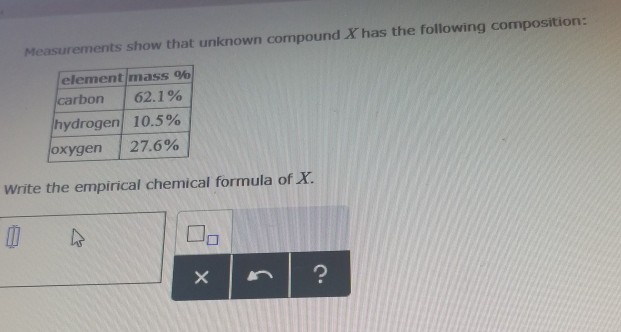
Solved Rank The Effective Nuclear Charge Z Experienced By A Chegg Com
Solved Calculate The Effective Nuclear Charge On A Valence Electron In An Oxygen Atom

Calculate Zeff For A Valence Electron In An Oxygen Atom Brainly Com
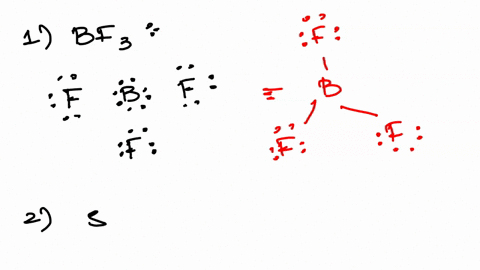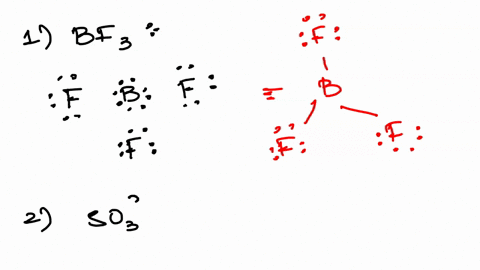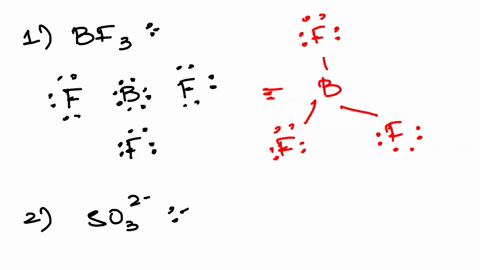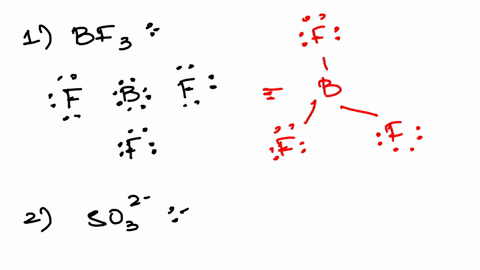
Solved Part A Calculate Zeff For A Valence Electron In An Oxygen Atom Express Your Answer Numerically View Available Hint S Azd Zeff 3 45 Submit Previous Answers Incorrect Try Again 4 Attempts Remaining

How To Use Slater S Rule To Estimate The Effective Nuclear Charge Youtube

Solved 6 Calculate Zeff For The Following A A Valence Chegg Com

Chapter 4 Periodic Trends Of The Elements Ppt Download
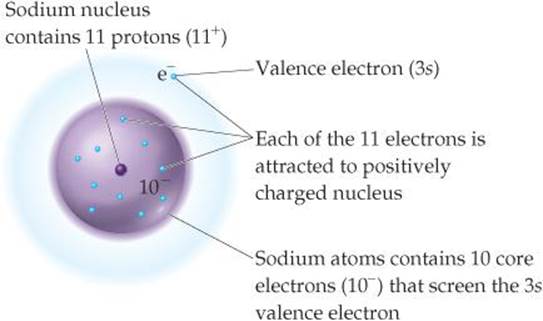
Effective Nuclear Charge Periodic Properties Of The Elements Chemistry The Central Science

The Effective Nuclear Charge For The Outermost Electron Of Oxygen Atom Is
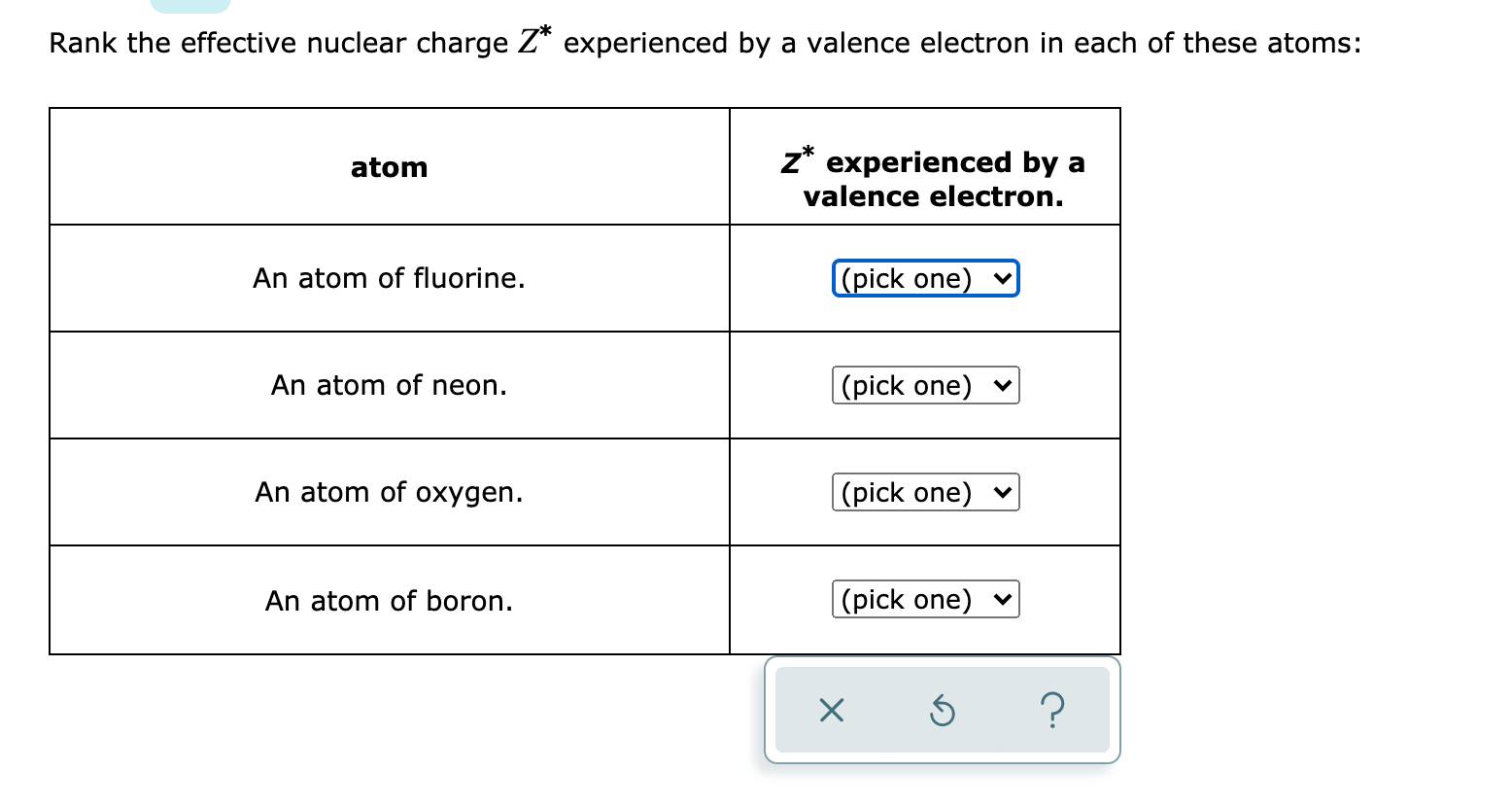
Answered Rank The Effective Nuclear Charge Z Bartleby

The Effective Nuclear Charge For Valence Electron In Underset 7 N 14 Will Be